Research Considers Returns on “Maximum Fair Price” of Medicare Drugs

A new working paper from the Center for Integration of Science and Industry at Bentley University shows that the National Institutes of Health (NIH) spent $11.7 billion on basic or applied research leading to approval of the ten drugs selected for Medicare price negotiations in the first year of the Inflation Reduction Act (IRA). The study, titled “Considering Returns on Federal Investment in the Negotiated 'Maximum Fair Price' of Drugs Under the Inflation Reduction Act: an Analysis” published by the Institute for New Economic Thinking, argues that the negotiated “maximum fair price” for these drugs must provide the public sector with returns on this federal investment comparable to the returns on private sector investment by the pharmaceutical companies.
The study showed that federal spending on research related to the discovery and development of these products represented a median investment cost of $895.4 million/drug and was comparable to reported industry investment in drug approvals over the same period. This NIH spending focused primarily on basic research. Application of this basic research by companies developing these products provides a median cost savings of $1,485 million/drug. Investment costs accounted for spending on the approved drugs, failed clinical trials, and discount rates or cost of capital as appropriate for public and private sector investment.
The study also shows that, from 2017-2021, Medicare Part D spent $126.3 billion (median $10.7 billion) (before rebates) to provide these ten products to 29.9 million beneficiaries. For eight of these drugs, representing $97.4 billion in Medicare Part D spending, these products provided beneficiaries with a total health benefit (total health value) of $67.7 billion or a net negative residual health value of -$29.6 billion.
Negotiated drug prices must provide equitable returns on both public and private sector investments
“This study emphasizes the co-creating roles of the public and private sector in pharmaceutical innovation and the essential investments made by both the public and private sector,” said Fred Ledley, Director of the Center for Integration of Science and Industry, and the senior author on this study. “The maximum fair price negotiated under the IRA must be one that not only makes these products available to patients with unmet medical needs, but also provides equitable returns on both federal and industry investment in drugs.”
The IRA authorizes the government to negotiate a “maximum fair price” for drugs with manufacturers based on factors including the manufacturer’s research and development costs, the returns on these investments, federal financial support for discovery and development, and the extent to which the drug addresses unmet medical needs. This study and an accompanying commentary “How Should the Government Negotiate Medicare Drug Prices? A Guide for the Perplexed” argue that the “maximum fair price” of these products should not be benchmarked to the total value associated with use of these products (the principle of value-based pricing), but rather to the margin (residual health value) that provides the social sector with an appropriate return on federal investment in these products (social return on investment).
Methods for identifying the contributions of NIH funding for basic and applied science to specific drug approvals have been described in a series of reports from the Center for Integration of Science and Industry examining public sector investment in pharmaceutical research and development.
Dr. Edward Zhou was the lead author of this work along with Dr. Paula Chaves da Silva, Debbie Quijada, and Dr. Fred Ledley. Ms. Quijada conducted this work as an undergraduate researcher at Bentley University. The Institute for New Economic Thinking provided funding for foundational studies on NIH funding for pharmaceutical innovation through a grant to Bentley University. The National Biomedical Research Foundation provides core funding for the Center for Integration of Science and Industry through a grant to Bentley University.
Public and private investment and returns related to Medicare Part D spending on drugs selected for Medicare price negotiation under the Inflation Reduction Act
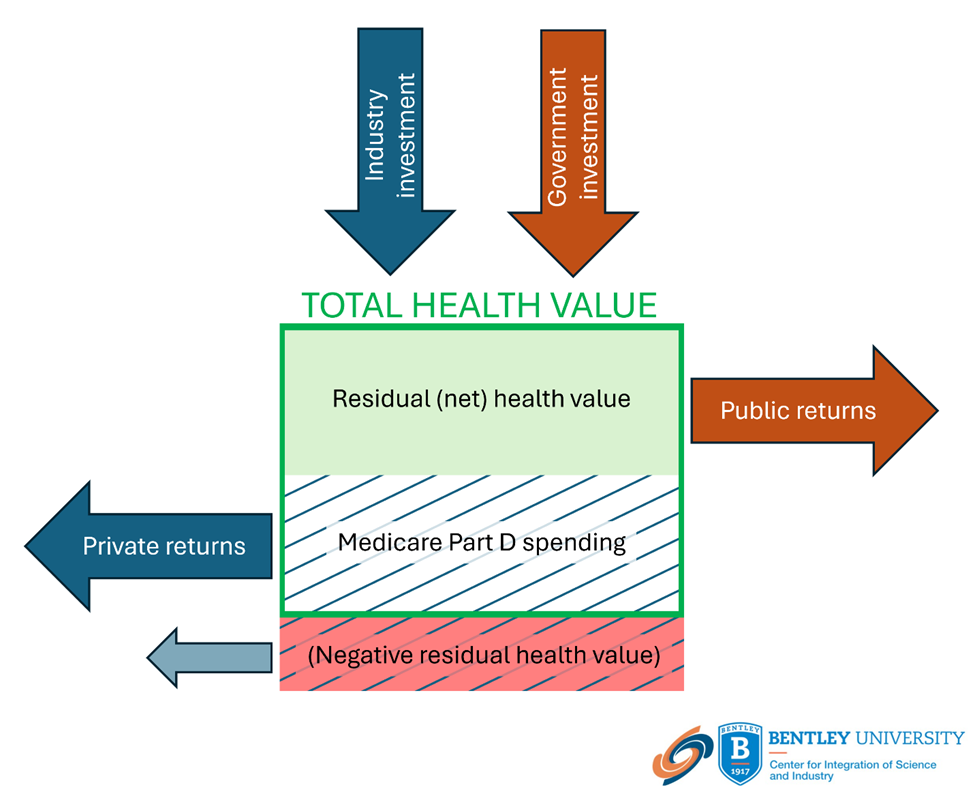
Investments by both industry and government are essential in discovering and developing new drugs. The value of these products is embodied in the total health value provided to those who use these products and is distributed between the private and public sectors by the price paid for the product and the residual (net) health value (surplus) to consumers. When Medicare Part D spending is less than the total health value (positive residual health value), both the public and private sectors realize returns.

